The SMS laboratory is equipped with the following thermal analysis devices (click on the links to know more):

Suplier: Netzsch ![]()
Principle: Analysis of the thermal behavior of samples by differential scanning calorimetry coupled with a thermogravimetric measurement and with a mass spectroscopy analysis of the chemical produced during the measurement.
Type of analyses performed: This apparatus measures sample mass loss as a function of temperature and analyzes the nature of the chemicals produced upon heating. Thus, the device is dedicated to the measurement of temperature of dehydration, desolvation, chemical degradation and is an important tool to design crystallization processes implying hydrates and solvates.
Highlights:

Suplier: Netzsch ![]()
Principle: Analysis of the thermal behavior of samples by differential scanning calorimetry. The device is equipped with an autosampler with 64 positions and is coupled with a liquid nitrogen system allowing sample analysis on a -150 500 °C temperature range.
Type of analyses performed: This device allows to measure enthalpies associated to phase transformations (polymorphic transitions, order disorder, melting, recrystallization...) or chemical reaction (degradations). The device is essentially used for the characterization of sub-ambient thermal behaviors.
Highlights:
Supplier: Mettler-Toledo & Olympus 

Principle: Thermal analyses by differential scanning calorimetry coupled with microscopy observations.
Type of analyses performed: This apparatus allows to measure enthalpies associated to phase transformations (polymorphic transitions, order-disorder transitions, fusion, recrystallization...). The thermal information is correlated with optical microscopy observations.
Supplier: Netzsch ![]()
Principle: Thermal analyses by differential scanning calorimetry. The device is equipped with a 20 positions sample tray and with an intracooler allowing analyses down to -70 °C.
Type of analyses performed: This apparatus allows to measure enthalpies associated to phase transformations (polymorphic transitions, order-disorder transitions, fusion, recrystallization...).
Supplier : Setaram ![]()
Funding organization : Région Normandie CaDi(2014) project. ![]()
Principle: The C80 calorimeter allows a high precision measurement of the heat emitted during temperature programs (isothermal or dynamic) thanks to a 3D Calvet sensor surrounding the sample. The attainable temperature range is from room temperature to 300 °C and heating rate ranges from 0.001 to 2 K/min.
Type of analyses performed: Standard Calorimetry : Standard stainless steel cells enable sensitive calorimetric measurements in isothermal and dynamic modes (heat capacity measurement of liquids and solids).
Reaction and Dissolution Calorimetry : Hastelloy mixture cells permit to reproduce chemical reactions, liquid/liquid mixtures and solid/liquid (even under mechanical stirring).
Detection of polymorphic phenomena by dissolution, investigation of the behavior of drugs under specific athmosphere, study of the cristallinity/ amorphous content of drugs, metabolism, thermal stability (application to pharmaceutical compounds).
Supplier: Prototype designed in the laboratory
Principle: DITA analyses allows to determine phase boundaries in phase diagrams (for instance the boundary between tri and biphasic domains).The system is placed in isoperibolic conditions (neither isothermal, nor adiabatic, but its direct environment is considered as perfectly isothermal).
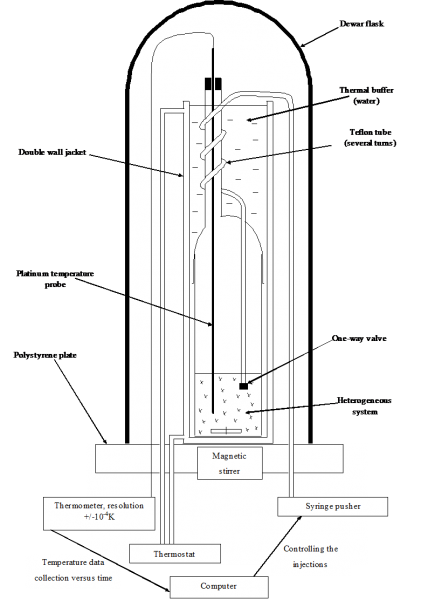
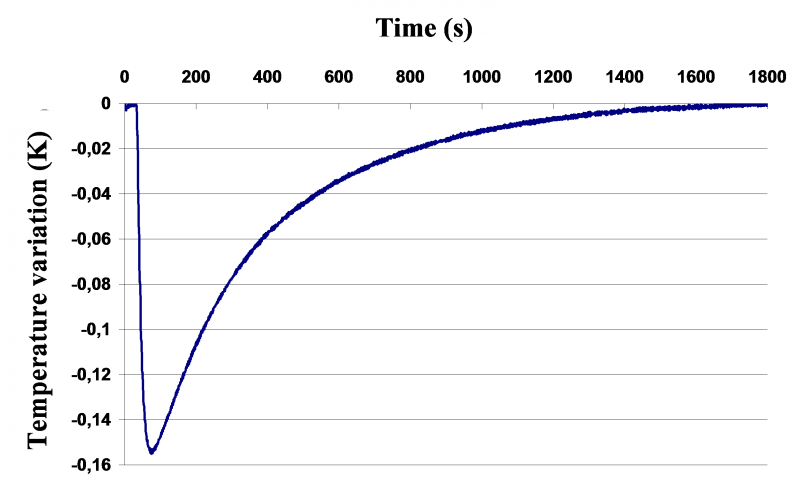
Schematic representation of the principle of the DITA prototype and typical T=f(V of injected solvent) curve
An aliquot amount of the solvent thermostated exactly at the same temperature as that of the initial suspension is quickly injected in the reactor. The thermodynamic phenomena involved (dissolution, dilution, crystallization, liquid/liquid demixing...) manifest by a temperature variation of the whole system (which is detectable down to 0.001 K - See Figure above - right).
The representation of the cumulative integral of the surface area of each T=f(V of injected solvent) (see Figure below) allows the interpretation of the system evolution during the analysis: each slope break corresponds to the appearence or the disapearence of at least one phase.
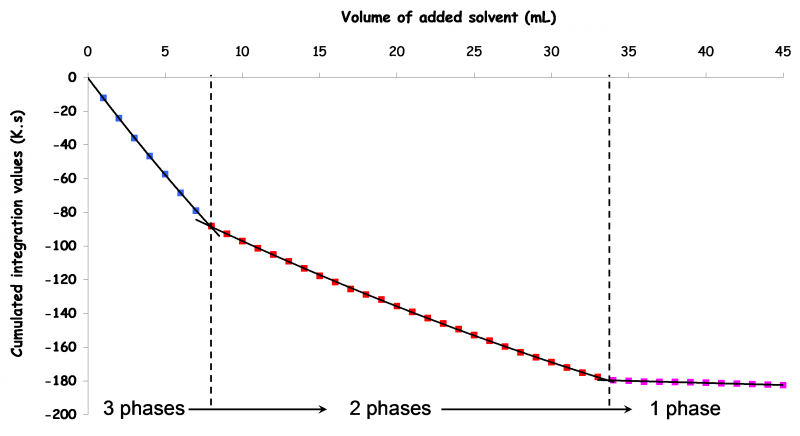
Exemple d'une opération DITA pour un système ternaire
For more information about DITA, you can contact us
Highlights:



