
The prototypes developed by the laboratory are:
- Sublimation furnace (secondary vacuum)
Supplier: Bruker AXS 
Principle: The -theta/-theta geometry of this device allows the in-situ analysis of solid phases in suspension in a liquid phase (500 mL reactor), or a solid in equilibrium with a vapor.
Type of analyses performed: This device is dedicated to the in-situ monitoring of crystallization processes in solution: the reactor temperature (from 70°C down to -30°C) and the antisolvent adding are controlled during the process and the system analyses the nature of the solid phase directly in suspension, which permits fo example to determine phase transition temperatures or peritectic temperatures (solvation, desolvation). The detection of efflorescent solvates becomes then a routine work. La détection de solvates efflorescents devient ainsi un travail de routine.
Highlights:

Supplier: 


Type of analyses performed:
-
Conglomerate spotting [1] and assessment of the non-centrosymmetry
-
Detection of solid impurity at the ppm level [2]
-
Study of polymorphic transitions (monotropic or enantiotropic [3], or order-disorder transitions [4]) implying at least one non-centrosymmetric phase.
-
Study of phase diagram implying at least one non-centrosymmetric phase. [5,6]
Highlights:
- A. Galland, V. Dupray, B. Berton, S. Morin-Grognet, M. Sanselme, H. Atmani and G. Coquerel, Cryst. Growth Des., 2009, 9, 2713–2718.
- S. Clevers, F. Simon, V. Dupray and G. Coquerel, J. Therm. Anal. Calorim., 2013, 112, 271–277.
- S. Clevers, F. Simon, M. Sanselme, V. Dupray and G. Coquerel, Cryst. Growth Des., 2013, 13, 3697–3704.
- S. Clevers, C. Rougeot, F. Simon, M. Sanselme, V. Dupray and G. Coquerel, J. Mol. Struct., 2014, 1078, 61–67.
- L. Yuan, S. Clevers, N. Couvrat, Y. Cartigny, V. Dupray and G. Coquerel, Chem. Eng. Technol., 2016, 39, 1326–1332.
Supplier: Prototype developped in the SMS laboratory
Principle: DITA analyses help to determine the boundaries between ajdcent domains in phase diagrams (for example, the boundary between a triphasic domain and a biphasic domain). The overall system is placed in isoperibolic conditions (neither isotherm, nor adiabatic, but its direct environment is considered as perfectly isotherm).
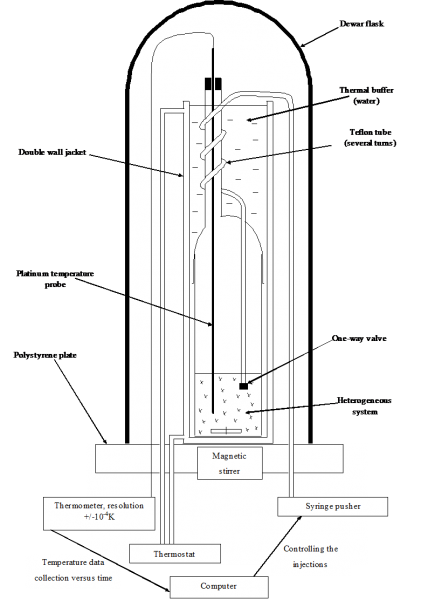
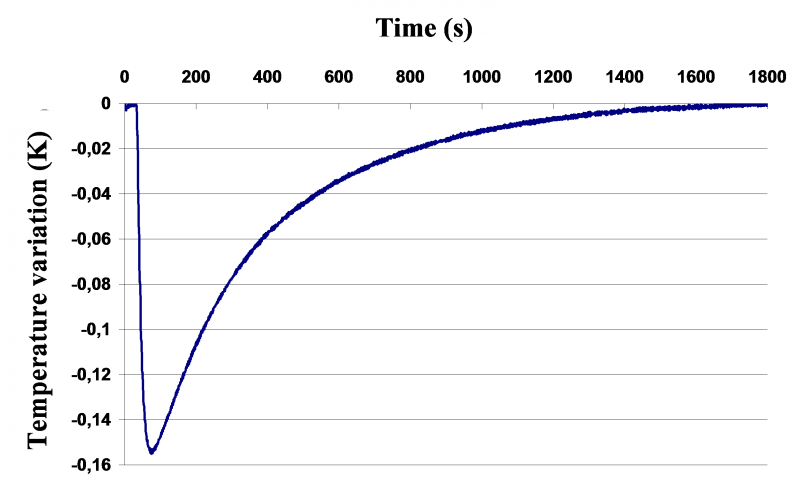
Schematic principle of DITA prototype (left) and typical recorded signal of temperature as a function of a solvent injection (right)
A defined quantity of solvent (strictly thermostated at the same temperature than that of the initial suspension) is rapidly injected in the reactor. Thermodynamic phenomena implied (dissolution, dilution, crystallization, demixing,...) will then be traduced by a temperature variation of the whole system (detection threshold at 0.001 K - see upper right figure).
Graphical representation of cumulated integrals of areas below every temperature variation curve as a function of the total amount of solvent added (see below) will help to interpret the evolution of the system upon the analysis: every slope change corresponds to the appeareance or the disappearance of at least one phase.
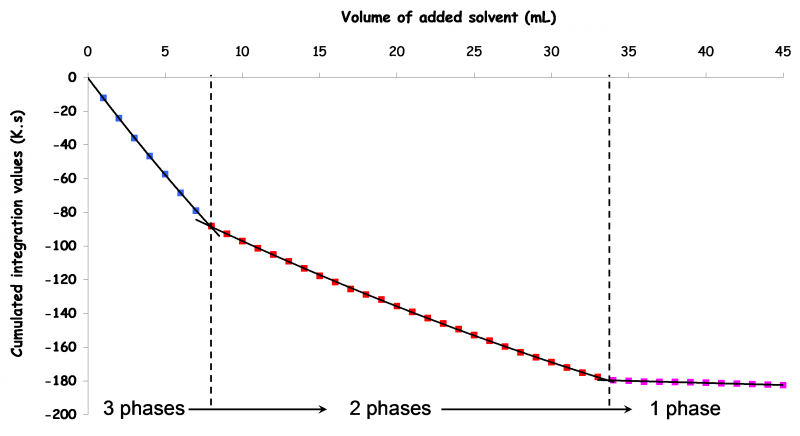
Exemple of DITA result for a ternary system
For more information, please contact directly the team
Highlights:

Supplier: 

Highlights:
Supplier: Lecordier Siverso, Cerhec, Edwards 


Principle: This device helps to purifiy volatile substances thermally stable: the purification acts through via a sublimation step and the separation is due to volatilities differences between the compounds. Recrystallization from the gaseous phase is controlled by a temperature gradient occurring inside a segmented tube in quartz. The experiment occurs under secondary vacuum (fixed, 10-10 bar).
Type of analyses performed: Purification to ultra-purification of thermally stable volatile compounds.



